10 Unbelievable Facts About Atoms That Make Science Fun
How often do you stop to think about the tiny building blocks of… well, everything? Atoms are the ultimate ghostwriters of reality—invisible, everywhere, and weirdly identical whether they’re in a distant star or your morning coffee. Yet most of us barely notice them unless we’re cramming for a science test.
Here’s the wild part: those same atoms—just reshuffled—make up both your smartphone and the Andromeda Galaxy. A carbon atom in your eyelash could’ve once been part of a dinosaur’s tooth. The oxygen you’re breathing? Recycled from some ancient cosmic explosion. It’s like the universe’s most chaotic Lego set, where 99.9% empty space somehow builds solid stuff.
Why does this matter? Because it’s proof that “ordinary” is a myth. Your taco, your dog, even Taylor Swift’s left pinky—all just temporary arrangements of particles that’ll someday rearrange again. Physics calls this atomic theory, but honestly, it feels more like magic.
Want your mind blown further? A single grain of sand contains quintillions of atoms. Yet they’re so small, if you enlarged one to marble size, the next atom would be a football field away. Most of what we “touch” is just electrons repelling each other—so technically, you’ve never actually touched anything.
Crazy, right? The universe is basically a billion-year-old chemistry experiment, and we’re all just walking through its latest iteration. Maybe atoms deserve a little more of our attention.
10. How Many Atoms Fit Across a Single Human Hair? Try Half a Million.

We often say something’s “a hair’s breadth away” to mean it’s incredibly close. But have you ever stopped to wonder how wide a human hair actually is? Not in millimeters or microns—but in atoms?
Brace yourself: the average human hair is roughly 500,000 atoms wide.
Yes, half a million atoms—lined up side by side—just to cross from one edge of a single strand of hair to the other. That tiny wisp of keratin you probably brushed aside this morning is a giant at the atomic scale.
To put it in context, the average atom measures about one angstrom across. An angstrom is one ten-billionth of a meter. That’s far smaller than the wavelength of visible light, which is why atoms are invisible to the naked eye—and even to most microscopes.
It wasn’t until 2004 that scientists developed a scanning transmission electron microscope with a resolution of 0.6 angstroms, finally allowing us to see individual atoms with stunning clarity. For comparison, your hair is about 500,000 to 1,000,000 times larger than this—depending on your hair type.
You pluck a hair or see one glinting in the sunlight, remember: you’re holding a structure made of trillions of atoms, stacked like microscopic building blocks. It’s a mind-bending reminder that even the most mundane parts of our body are made from unimaginably tiny pieces of the universe.
9. Your Body Contains More Atoms Than the Entire Universe Has Stars—Yes, Really
Let’s start with something tiny: a single strand of human hair. It’s made of roughly 500,000 atoms side by side. Now, zoom out. Your entire body? A mind-blowing 6.5 octillion atoms—that’s a 6.5 followed by 27 zeros. To put that in perspective, the observable universe has “only” about 200 billion trillion stars. So, congratulations—you’re basically a walking galaxy.
But Wait, It Gets Weirder
About 96% of you is just four elements: carbon, oxygen, hydrogen, and nitrogen. The rest? A sprinkle of calcium, traces of gold, and even some copper—like a cosmic smoothie blended over billions of years. And here’s the kicker: your atoms aren’t even loyal. You’re constantly swapping them out—breathing, eating, shedding skin. Over a lifetime, you’ll cycle through enough atoms that you’ve probably inhaled a few from Shakespeare’s last breath or sweated out particles once trampled by dinosaurs.
Why Can’t We Wrap Our Heads Around This?
Because numbers like “octillion” are nonsense to human brains. Imagine counting to a billion—it’d take 31 years. Now try octillion. Yeah, didn’t think so. The universe’s 200 billion trillion stars? That’s already an absurd abstraction. But your atoms outnumber them all, which means you’re literally more complex (by count) than the cosmos.
The Takeaway? You’re Stardust with a To-Do List
Every atom in your hand could’ve been forged in a supernova, recycled through oceans, or even passed through Cleopatra’s lungs. So next time you feel small, remember: your body contains a universe’s worth of raw material—just rearranged into something that binge-watches Netflix and forgets passwords. Not bad for a pile of cosmic leftovers.
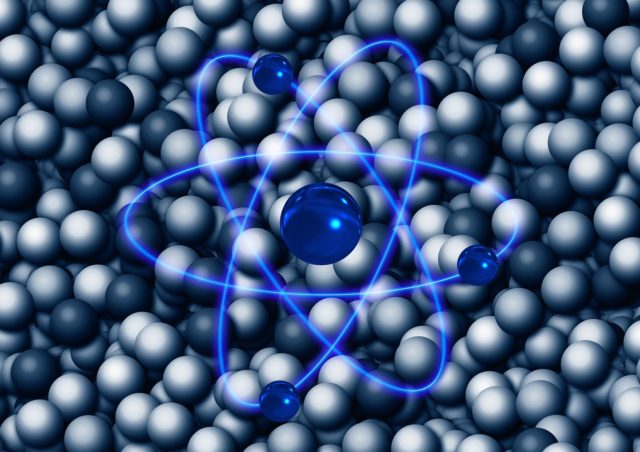
8. How Big Is an Atom Compared to Its Nucleus? Think Stadium vs. Blueberry.
Atoms are mind-bendingly small. So small, in fact, that most of us just shrug and go about our day—because what does atomic scale really have to do with walking the dog or making spaghetti?
But when you stop and zoom in—really zoom in—what you find is absolutely wild.
At the heart of every atom is a nucleus, a tiny core made up of protons and neutrons. Orbiting that nucleus are electrons—billions upon billions of them in all the stuff around you. So how much smaller is the nucleus compared to the entire atom?
Here’s a jaw-dropper:
If the atom were the size of a football stadium, the nucleus would be about the size of a blueberry.
Or, if baseball is more your thing:
A stadium-sized atom has a nucleus no bigger than a ping pong ball.
In actual numbers?
An atom is roughly 100,000 times larger than its own nucleus.
This means the vast majority of an atom—of you, your phone, the air you breathe—is just empty space. That’s not poetic exaggeration. That’s physics.
So someone tells you they need space, you can remind them:
“We’re already mostly space.”
7. You Might Share 200 Billion Atoms with William Shakespeare (Seriously)

Ever wondered how connected you really are to history? Here’s a thought that might just blow your atomic mind:
You may share up to 200 billion atoms with William Shakespeare.
Yep—the William Shakespeare. Poet. Playwright. Possibly your atomic roommate.
It sounds far-fetched, but it’s actually not. Here’s why:
Most of the atoms in your body are hydrogen, followed by oxygen and carbon. These atoms don’t disappear when someone dies—they cycle through the environment. They float in the air, dissolve in water, get absorbed by plants, eaten by animals, and—eventually—by you.
Shakespeare breathed, ate, and sweated just like anyone else. His atoms scattered over the centuries through air, water, and earth, becoming part of the natural cycle. Every breath you take pulls in trillions of molecules—some of which once passed through Shakespeare’s lungs. That drop of water in your tea? It might’ve once been part of his sweat—or the wine he toasted with.
Researchers suggest that due to the sheer number of atoms and the way they constantly move and recycle, it’s completely plausible that you now contain hundreds of billions of the same atoms that once made up the Bard himself.
And not just Shakespeare.
You likely share atoms with every human being who has ever lived.
From Cleopatra to Confucius, from Einstein to your great-great-great-grandparents—you’re literally made of the past.
6. Your Body Replaces 98% of Its Atoms Every Year—You’re Basically a Quantum Ship of Theseus
You think you’re you? Think again. On an atomic level, you’re more like a river than a statue—constantly flowing, changing, and rebuilding. Here’s the wild part: 98% of your atoms get swapped out every year. That’s right—the “you” from 12 months ago is almost entirely gone, replaced by a nearly identical (but technically brand-new) version.
How Does This Atomic Shuffle Even Work?
- Your skin sheds 40,000 cells per minute—that’s about 9 pounds of dead skin per year, all replaced by fresh atoms.
- Your bones? Even their sturdy carbon framework gets a 50% refresh every few months.
- Your blood fully regenerates every 4 months, meaning the iron in your veins today wasn’t there last summer.
- Every breath swaps out oxygen atoms, every meal rebuilds your tissues, and every sip of water flushes out old hydrogen.
You’re Literally Made of Other People (Including Shakespeare)
With 6 octillion atoms in your body and a 98% annual turnover, you’re cycling through 5.88 octillion new atoms yearly—many of which once belonged to:
- Historical figures (yes, you’ve probably inhaled a few of Einstein’s exhaled atoms).
- Dinosaurs, ancient oceans, and extinct stars (thanks, cosmic recycling program).
- Every person you’ve ever shared a room with (gross? Poetic? Both?).
Why This Blows Your Mind (Even If You Can’t Feel It)
- Your “permanent” body is a myth—you’re more like a pattern than a fixed object.
- You’re atomically entangled with all of humanity—your breath right now contains nitrogen from Genghis Khan’s final sigh.
- You’ve “been” everywhere—atoms in your left pinky might’ve once been part of a Martian meteorite or a blue whale’s heart.
Bottom Line: You’re not just in the universe—you’re a temporary gathering of its oldest ingredients, borrowing stardust until it’s time to pass it on. So next time someone says “you’ve changed,” just wink and say, “Literally.”
5. You Literally Breathe Out Fat—And It’s More Fascinating Than You Think

We hear it all the time: “burn calories, lose weight.” But let’s be honest—what does that actually mean? Where does the fat go? Does it melt? Does it evaporate? Does it get flushed away?
The truth is way more surprising, and a little mind-blowing.
When your body burns fat, it doesn’t magically disappear. It has to go somewhere. And spoiler alert: it doesn’t leave through sweat alone. In fact, most of it escapes through your breath.
Yep, that’s right. You literally exhale your fat.
Here’s how it works: your body stores fat in molecules called triglycerides, made up of carbon, hydrogen, and oxygen. When you exercise or start using more energy than you consume, your body breaks these fat molecules apart. This breakdown process releases energy, and what’s left behind is transformed—some into water, but the majority into carbon dioxide.
And where does that CO₂ go?
Out through your mouth and nose, every time you exhale.
To get a clearer picture, imagine this: if you lose 10 pounds, around 1.6 pounds of that leaves your body through fluids—like sweat, urine, or even tears. But the other 8.4 pounds? You’re literally breathing it out over time. Quietly, invisibly—fat molecules leaving your body with every breath.
So yes, when your fitness coach tells you to remember to breathe, they’re more right than you thought.
It’s not just about oxygen delivery—it’s about getting rid of waste. And fat is waste, once your body has converted it to fuel.
This process, surprisingly elegant and completely invisible, happens every moment your metabolism is humming along. Even while you’re sleeping. Even right now.
Want to gain that weight back? You’d need to eat or drink enough carbon again to rebuild those fat stores. That’s why fat loss isn’t about sweating bullets or starving yourself—it’s about creating the right conditions for your body to do what it already knows how to do.
And maybe now you’ll never look at breathing the same way again.
4. Half of You Isn’t Even From This Galaxy—Literally

Think you’ve never left Earth? Think again. You, yes you, are a cosmic traveler—whether you realize it or not. Because roughly half of the atoms in your body didn’t even come from the Milky Way.
Let that sink in.
Every cell in your body is built from atoms—tiny particles that were forged in the cores of ancient stars, billions of years before Earth was even a thing. But here’s the truly mind-bending part: around 50% of those atoms came from entirely different galaxies. You’re walking around with a cosmic cocktail of matter that’s older and more well-traveled than anything on Earth.
So how did this happen?
When massive stars explode in supernovas, they scatter unimaginable amounts of particles into the universe. Some of that star-stuff drifts across the void, pulled by gravity, carried by galactic winds, and eventually sucked into neighboring galaxies—like ours. Over billions of years, those atoms mixed with the dust and gas that formed our solar system, our planet, and ultimately… us.
That means every time you blink, breathe, or brush your teeth, you’re using atoms that have journeyed across intergalactic space. Kinda makes your morning coffee feel a bit more profound, doesn’t it?
You are, quite literally, part alien—not in a sci-fi, green-skin kind of way, but in the most profound sense. Half of what makes you you is forged far beyond the limits of our galactic neighborhood. And that’s not just poetic—it’s real, measurable science.
3. Graphene: The One-Atom-Thick Wonder Material That Defies Imagination
Let’s shrink the scale from octillions of atoms to something far simpler—yet no less astonishing: a single layer of atoms. That’s graphene, the ultra-thin, ultra-powerful material that’s been revolutionizing science for over a decade.
What Makes Graphene So Mind-Blowing?
- It’s just one atom thick—picture a sheet of carbon atoms arranged in a honeycomb lattice, so thin it’s practically 2D.
- Stronger than steel, tougher than diamond—yet flexible enough to bend like plastic.
- Conducts electricity better than copper—making it a game-changer for ultra-fast electronics.
“Wait… How Can Something One Atom Thick Be Useful?”
Great question. In 2008, scientists created a graphene “balloon”—just one atom thick—that could trap gas inside. Wrap your head around that: a sheet a million times thinner than a human hair, yet strong enough to hold molecules in place.
Why Isn’t Graphene Everywhere Yet?
- Production is tricky—making large, flawless sheets is still expensive and slow.
- We’re figuring out applications—from unbreakable phone screens to lightweight space elevators, the possibilities are endless but still in development.
The Future? A World Built on Atom-Thin Tech
Imagine:
- Computers 100x faster with graphene chips.
- Unbreakable, paper-thin armor for vehicles.
- Medical sensors embedded in your skin like temporary tattoos.
One day, graphene might be as common as plastic—but for now, it’s a glimpse into a future where the thinnest material is also the strongest.
2. How Many Atoms Are in the Universe? Get Ready for a Number That’ll Break Your Brain

So you think you’ve heard some big numbers before? Maybe a billion, maybe a trillion. Maybe you’ve even dared to imagine a googol. But when it comes to the number of atoms in the universe, we’re dealing with something that makes all those numbers feel embarrassingly small.
Let’s talk absurd scale.
Scientists have taken a crack at estimating just how many atoms exist in the known universe (emphasis on known, because what lies beyond that is still cosmic mystery). Using estimates about the number of galaxies, the stars in each galaxy, and the stuff—matter—that makes up all of it, the math gets, well, out of hand.
And what they came up with? A number so staggeringly massive it doesn’t even sound real: somewhere between 10 quadrillion vigintillion and 100,000 quadrillion vigintillion atoms.
No, that’s not a typo. And yes, vigintillion is a real word. That’s 10⁷⁸ to 10⁸² atoms. Imagine a 1 followed by 82 zeroes. Go ahead, try to picture it. You can’t. None of us can. Our brains were not built to handle that kind of numerical insanity.
Want a relatable comparison? If you counted one atom per second, non-stop, with no sleep or breaks, it would take you far longer than the age of the universe just to reach the smallest end of that estimate.
And that’s just the visible universe. The stuff we can detect. The actual number might be way higher if the universe turns out to be bigger—or infinite.
It’s one of those facts that hits hard: You, your phone, your cat, every star, every drop of water, every planet, every black hole, every molecule of air—it’s all made of atoms. And yet they’re still outnumbered by the total atoms floating across the cosmos by, well, an amount we literally can’t imagine.
1. Humanity in a Sugar Cube? The Strange Truth About How Empty You Really Are

You probably don’t feel like a floating cloud of nothingness—but scientifically, that’s pretty much what you are. Every person, no matter how big or small, is mostly made of empty space. And if that doesn’t sound wild enough, here’s the kicker: if you removed all the empty space from the atoms in every human on Earth, the entire human race would fit inside a single sugar cube.
Let’s break it down.
The average person is built from about 6 octillion atoms (that’s a 6 followed by 27 zeros). Now imagine this person—say, a 200-pound, 6’2″ guy. His atoms, and yours, are mostly nothing. Each atom has a tiny nucleus at the center, surrounded by a cloud of electrons. But here’s the twist: the nucleus is about 100,000 times smaller than the atom itself. That’s like putting a blueberry in the middle of a football stadium and calling the whole thing an atom.
And it’s not just the space inside atoms. There’s space between molecules, and between the atoms that make up those molecules. All matter—no matter how solid it feels—is mostly made of vacuum.
What happens if we hypothetically remove all that space from all 8 billion people on Earth?
What remains would be a ridiculously dense clump of pure nuclear material, no bigger than a sugar cube, yet still weighing as much as all of humanity combined—hundreds of millions of tons. You couldn’t pick it up, of course. You couldn’t even stand near it safely. It would be as dense and deadly as a neutron star fragment.
This strange but true idea comes from the fact that 99.9999999% of matter is empty space. We seem solid, but in reality, we’re more like walking illusions held together by invisible atomic forces. Strip away that space, and what’s left would barely take up room in your pocket.

























